April 13, 2021 Print
For the first time, researchers at The Westmead Institute for Medical Research (WIMR) have identified two subtypes of Mononuclear Phagocyte (MNP) cells that play important roles in detecting then transmitting HIV virus. Importantly these cells play a vital role in switching on an immune response. This discovery is an important step in identifying cells that could be targeted with a HIV vaccine.
This study, published in
Nature Communications is a continuation of previous work published by the WIMR team, focusing on the important role of MNPs in instigating an immune response to HIV.
Post-doctoral researcher and first author on this study, Jake Rhodes, says that MNPs in human tissue are specialised at detecting a pathogen and delivering it to the primary target cells in the immune system.
He explains, “In the case of HIV, MNPs detect and deliver the virus to CD4T cells, which then help to coordinate an immune response by stimulating other cells to fight the virus.
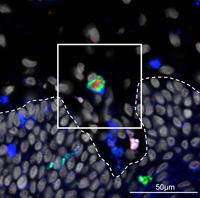
“Most studies looking at the role of MNPs in the transmission of HIV have focused on MNPs located at the epithelial level – the thin tissue that forms the outer layer of the body. However, trauma and inflammation of the mucous membrane are known to be strongly associated with HIV transmission, so our team examined the role of sub-epithelial MNPs, located in the underlying deeper tissue layers.”
Second author on this study, Dr Kirstie Bertram explains that, for this study, the WIMR team used actual anogenital tissue samples, donated post-surgery for research purposes. “The cells in these tissues behave differently to cells in other parts of the body, so being able to use actual tissue samples was vital.
“Our research showed that HIV can penetrate the epithelial surface to interact with sub-epithelial MNPs in anogenital tissue samples. Using advanced technologies, our team was able to watch as these MNPs took up HIV within 30 minutes of exposure to the virus.”
The team were able to define the full range of MNP subsets that are present in human anogenital and colorectal tissues, and that encounter HIV during sexual transmission of the virus.
As a result, the team was able to identify two MNP subsets that take up the HIV pathogen, become infected, and then transmit the virus to CD4T cells. The subsets identified are:
• CD14+CD1c+ monocyte-derived dendritic cells
• Langerin-expressing conventional dendritic cells 2.
A senior researcher on this study, Associate Professor Andrew Harman says that, compared with other MNPs, both subsets took up HIV more efficiently and became more infected.
“We found that CD14+CD1c+ monocyte-derived dendritic cells were most efficient at transferring HIV to CD4T cells at late time points. However, we discovered that Langerin-expressing conventional dendritic cells 2 transferred the virus more effectively at early time points.
“These findings are important because, to develop a HIV vaccine, we need to understand which dendritic cell subsets pick-up HIV and switch on the immune response. A vaccine would target these subsets of cells,” says Associate Professor Harman.
There is no vaccine or cure for HIV/AIDS. A HIV vaccine is vital to reducing the impact of HIV which is now, in most cases, transmitted sexually. With around 37 million people infected globally, and 1.8 million new infections each year, finding a vaccine remains a priority for researchers.
While antiretroviral therapy (ART) is efficient at controlling HIV infection, it is a lifelong treatment which is costly and is associated with toxicities. Only 57% of HIV positive individuals receive ART.
Associate Professor Harman says, “ART can also be given to healthy ‘at risk’ individuals as pre-exposure prevention, and this has been shown to be effective in reducing transmission. However, this is not a global solution due to lack of access in low income countries, and variable uptake in Western countries. In addition, preventative treatment has recently been shown to be ineffective in situations where the mucous membrane is inflamed, as is normally the case with sexual transmission of HIV.”
The WIMR team’s next steps will seek to further understand the mechanisms by which these MNP cells interact with HIV.
Jake Rhodes says, “Our next steps are to define the precise receptors that these cells use to bind HIV, with the aim of blocking the transmission of HIV and these receptors. We will also seek to identify exactly how these cell types activate the immune system, and what they do once they have encountered HIV.”
* IMAGE: Langerin+ cDC2 taking up HIV in inner human foreskin after 2 hours. Green = Langerin, Blue = CD11c, Red = HIV. Dotted line outlines the basement membrane separating the epidermis (bottom) from the dermis (top).